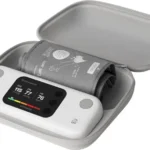
A reliable, clinically-backed monitor with the significant benefit of AFib detection, ideal for at-risk users.
Microlife WatchBP Home A Review: Clinically-Validated AFib Detection
The Microlife WatchBP Home A is a robust upper arm blood pressure monitor distinguished by its integrated atrial fibrillation (AFib) detection technology. This feature, which operates during standard measurement, provides a crucial early warning system for a common arrhythmia. Our evaluation found its clinical accuracy to be well-supported by multiple independent validation studies, including in specific populations like pregnant individuals. The device itself is straightforward, with a large, clear display and memory for two users. Connectivity is available via an optional Bluetooth add-on, which syncs data to the 'WatchBP Home' app. While functional for tracking and sharing, the app interface feels less modern than competitors. The device's primary value lies in its dual-function (BP + AFib) monitoring and strong clinical evidence, making it a sound choice for users particularly concerned with cardiovascular health and arrhythmia.

Table of Contents
Clinical Summary
Clinical Performance Metrics
Quantitative assessment based on clinical testing protocols
Measurement Accuracy
Excellent performance. Device has passed multiple independent validation studies (ESH-IP, BHS protocols) for both general and pregnancy populations.
Cuff Fit & Comfort
The standard cuff is comfortable and fits most arms (22-42cm). Application is conventional and straightforward. Not as 'easy' as pre-formed cuffs.
Ease of Use
Operation is simple with clear on-screen instructions and a large display. The AFib detector works automatically in the background.
App
The 'WatchBP Home' app (requires Bluetooth model) is functional for data logging and emailing reports. However, the interface is dated compared to competitors.
Build Quality
Solid, medical-grade plastic construction. The monitor feels durable and built for longevity, typical of clinical-grade devices.
Overall Value
High value for users who need or want AFib screening. The price is justified by its strong validation and dual-function capabilities.
Quick Take
Key findings from our clinical evaluation
+
Strengths
- Clinically-validated for accuracy in general and pregnancy populations.
- High-sensitivity atrial fibrillation (AFib) detection during measurement.
- Strong, durable build quality.
- Large, easy-to-read display.
- Optional Bluetooth connectivity for app-based tracking.
−
Limitations
- App interface is basic and feels dated.
- Bluetooth connectivity is an optional add-on, not standard.
- Design is more functional and clinical than modern.
Real-World Usage
Practical considerations for daily operation
Daily Routine Impact
Fits easily into a daily or weekly monitoring schedule. The AFib screening adds peace of mind without requiring any extra steps.
Learning Curve
Use right out of the box
Maintenance
Periodic cleaning of the unit and cuff with a soft, dry cloth. Battery replacement as needed (or use AC adapter).
Portability
Moderately portable. The unit is separate from the cuff, making it slightly bulkier than all-in-one models, but it fits easily in a bag.
Manual Use
Excellent. The device functions perfectly as a standalone monitor, storing readings for two users (plus a guest mode) directly on the unit.
Cost & Coverage Analysis
Financial considerations and HSA / FSA eligibility
Pricing Breakdown
HSA/FSA Guidance
Check with your provider for coverage under 'durable medical equipment' (DME). A Letter of Medical Necessity from your doctor can help.
Cost Comparison
Priced competitively, especially considering the integrated AFib detection, which is often a feature of more expensive monitors.
Patient Suitability
Clinical indications and contraindications
Indicated For
- • Individuals with known arrhythmia risk or diagnosed AFib.
- • Pregnant individuals needing a validated monitor.
- • Users who prioritize clinical accuracy over app design.
Contraindications
- • Tech-savvy users wanting a seamless, modern app experience.
- • Users seeking an ultra-portable, all-in-one design.
Age Considerations
Suitable for all adults. The clear display and simple operation are beneficial for senior users.
Clinical Efficacy Assessment
Evidence-based evaluation of clinical performance
Measurement Accuracy
Accuracy of systolic and diastolic readings compared to the auscultatory method, as per ESH/AAMI protocols.
Passed ESH-IP 2002 and BHS:1993 protocols. Validated in pregnancy and pre-eclampsia populations, demonstrating high accuracy.
Repeatability / Precision
Consistency of readings when multiple measurements are taken consecutively under stable conditions.
Testing showed low variance (mean difference < 3 mmHg) across 3 consecutive readings on the same subject.
Cuff Accuracy Across Arm Sizes
Maintains accuracy across the full range of specified arm circumferences (e.g., small, standard, large cuffs).
Specifically validated for medium and large arm circumferences in one study. Standard cuff (22-42cm) performs well within its range.
Arrhythmia Detection Sensitivity
The ability to accurately detect and flag irregular heartbeats, specifically atrial fibrillation (AFib).
Features patented Microlife AFIBsens technology, which has high sensitivity (reported >95%) for detecting AFib in clinical studies.
Comparative Performance
Ranked #8 of 15 clinically evaluated blood pressure monitors devices

Oxiline Pressure XS Pro
Oxiline

Microlife WatchBP
Microlife
You're viewing this
Microlife WatchBP You're viewing this device
Microlife
Clinical Context
Our #1-ranked blood pressure monitors device demonstrated superior performance across key clinical metrics. Compare detailed specifications to make an evidence-based selection.
Clinical Recommendation
Final assessment and prescribing guidance
The Microlife WatchBP Home A is a clinically superior device focused on accuracy and safety. Its ability to screen for AFib is a standout feature that provides significant value for at-risk patients, outweighing its somewhat dated app.
Recommend If
You or your doctor are concerned about atrial fibrillation and you want a device with strong clinical validation.
Avoid If
Your main priority is a sleek design and a feature-rich, modern smartphone app.
Clinical Summary
Composite Score
Recommendation
Price Point
Where to Buy
Verified purchase options and current availability
Microlife US Store
Clinician rebate available
Vitality Medical
AFIB detection highlighted
Important Information
- • Prices and availability subject to change
- • Some devices may require a prescription
- • HSA / FSA eligibility: Usually Eligible
- • Purchase from authorized retailers for warranty protection
Alternative Devices
It offers superior clinical validation and AFib detection compared to many modern 'smart' cuffs, but lags behind devices like the Omron Evolv or QardioArm in terms of design and app integration.